Evaluate results with/without noisy training labels
Joyce Hsiao
- Extract data from the top 10 genes identified
- Fitting using top 10 genes before/after removing PC outliers
- fitting top 10 genes without PC outliers
- Cell time properties before/after removing PC outliers (intensity PVE)
- Re-fitting after removing PC outliers from the same 5 folds, top 101 genes
- Fitting including PC outliers
- Session information
Last updated: 2018-07-14
Code version: a282d43
Extract data from the top 10 genes identified
library(Biobase)
library(circular)
source("../peco/R/cycle.corr.R")
source("../peco/R/cycle.npreg.R")
df <- readRDS(file="../data/eset-final.rds")
pdata <- pData(df)
fdata <- fData(df)
# select endogeneous genes
counts <- exprs(df)[grep("ENSG", rownames(df)), ]
log2cpm.all <- t(log2(1+(10^6)*(t(counts)/pdata$molecules)))
# select external validation samples
log2cpm.quant <- readRDS("../output/npreg-trendfilter-quantile.Rmd/log2cpm.quant.rds")
set.seed(99)
nvalid <- round(ncol(log2cpm.quant)*.15)
ii.valid <- sample(1:ncol(log2cpm.quant), nvalid, replace = F)
ii.nonvalid <- setdiff(1:ncol(log2cpm.quant), ii.valid)
log2cpm.quant.nonvalid <- log2cpm.quant[,ii.nonvalid]
log2cpm.quant.valid <- log2cpm.quant[,ii.valid]
sig.genes <- readRDS("../output/npreg-trendfilter-quantile.Rmd/out.stats.ordered.sig.101.rds")
expr.sig <- log2cpm.quant.nonvalid[rownames(log2cpm.quant.nonvalid) %in% rownames(sig.genes)[1:10], ]
# get predicted times
# set training samples
source("../peco/R/primes.R")
source("../peco/R/partitionSamples.R")
parts <- partitionSamples(1:ncol(log2cpm.quant.nonvalid), runs=5,
nsize.each = rep(151,5))
part_indices <- parts$partitionsFitting using top 10 genes before/after removing PC outliers
# first check the theta in pdata
pca <- prcomp(cbind(pdata$rfp.median.log10sum.adjust,
pdata$gfp.median.log10sum.adjust), scale=TRUE)
pca_df <- cbind(pca$x[,1],pca$x[,2])
rownames(pca_df) <- rownames(pdata)
theta_check <- as.numeric(coord2rad(pca_df))
theta_check <- 2*pi-theta_check
plot(theta_check, pdata$theta)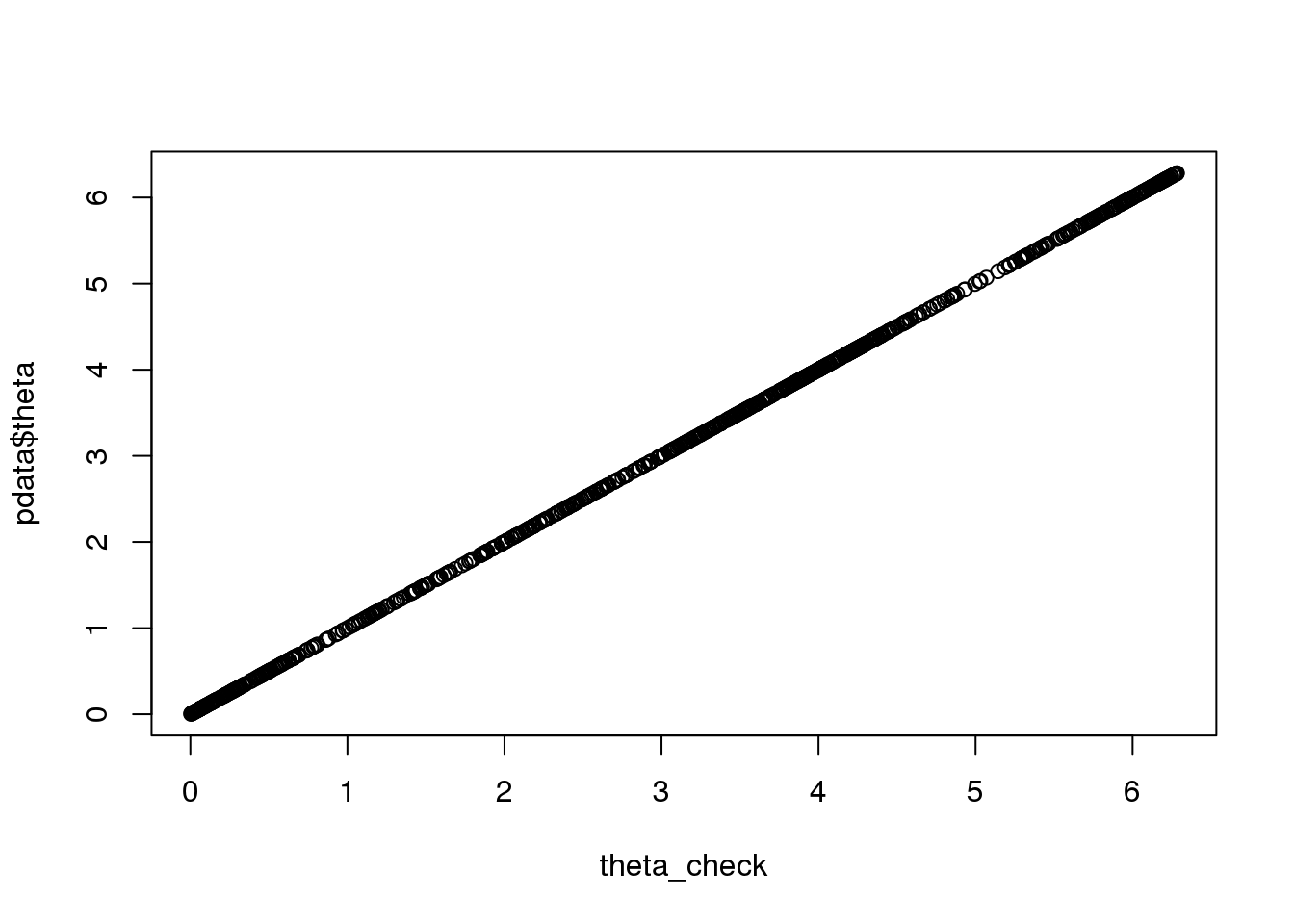
names(theta_check) <- rownames(pdata)fits_top10 <- vector("list", 5)
for (run in 1:5) {
print(run)
# fitting training data
Y_train <- expr.sig[,part_indices[[run]]$train]
theta_train <- theta_check[match(colnames(Y_train), rownames(pdata))]
names(theta_train) <- colnames(Y_train)
fit.train <- cycle.npreg.insample(Y = Y_train,
theta = theta_train,
ncores=12,
method.trend="trendfilter")
# fitting test data
Y_test <- expr.sig[,part_indices[[run]]$test]
theta_test <- theta_check[match(colnames(Y_test), rownames(pdata))]
names(theta_test) <- colnames(Y_test)
fit.test <- cycle.npreg.outsample(Y_test=Y_test,
sigma_est=fit.train$sigma_est,
funs_est=fit.train$funs_est,
method.grid = "uniform",
method.trend="trendfilter",
polyorder=2,
ncores=12)
fits_top10[[run]] <- list(fit.train=fit.train,
fit.test=fit.test,
theta_test=theta_test)
}
for (i in 1:5) {
fits_top10[[i]]$theta_est_shift <- rotation(fits_top10[[i]]$theta_test, fits_top10[[i]]$fit.test$cell_times_est)$y2shift
}
saveRDS(fits_top10, file = "../output/method-train-labels.Rmd/fits_top10.rds")
#fits_top10 <- readRDS(file = "../output/method-train-labels.Rmd/fits_top10.rds")
source("../peco/R/utility.R")
diff_time <- lapply(1:length(fits_top10), function(i) {
pmin(abs(fits_top10[[i]]$theta_est_shift-fits_top10[[i]]$theta_test),
abs(fits_top10[[i]]$theta_est_shift-(2*pi-fits_top10[[i]]$theta_test)))
})
pve <- lapply(1:length(fits_top10), function(i) {
dapi <- pdata$dapi.median.log10sum[match(names(fits_top10[[i]]$theta_test),rownames(pdata))]
get.pve(dapi[order(fits_top10[[i]]$theta_est_shift)])
})
save(diff_time, pve,
file="../output/method-train-labels.Rmd/modelresults_top10.rda")load(file="../output/method-train-labels.Rmd/modelresults_top10.rda")
mean(sapply(diff_time, mean)/2/pi)[1] 0.08688909mean(unlist(pve))[1] 0.2419089explore PCA outliers
# first check the theta in pdata
pca <- prcomp(cbind(pdata$rfp.median.log10sum.adjust,
pdata$gfp.median.log10sum.adjust), scale=TRUE)
pca_df <- cbind(pca$x[,1],pca$x[,2])
rownames(pca_df) <- rownames(pdata)
theta_check <- as.numeric(coord2rad(pca_df))
theta_check <- 2*pi-theta_check
plot(theta_check, pdata$theta)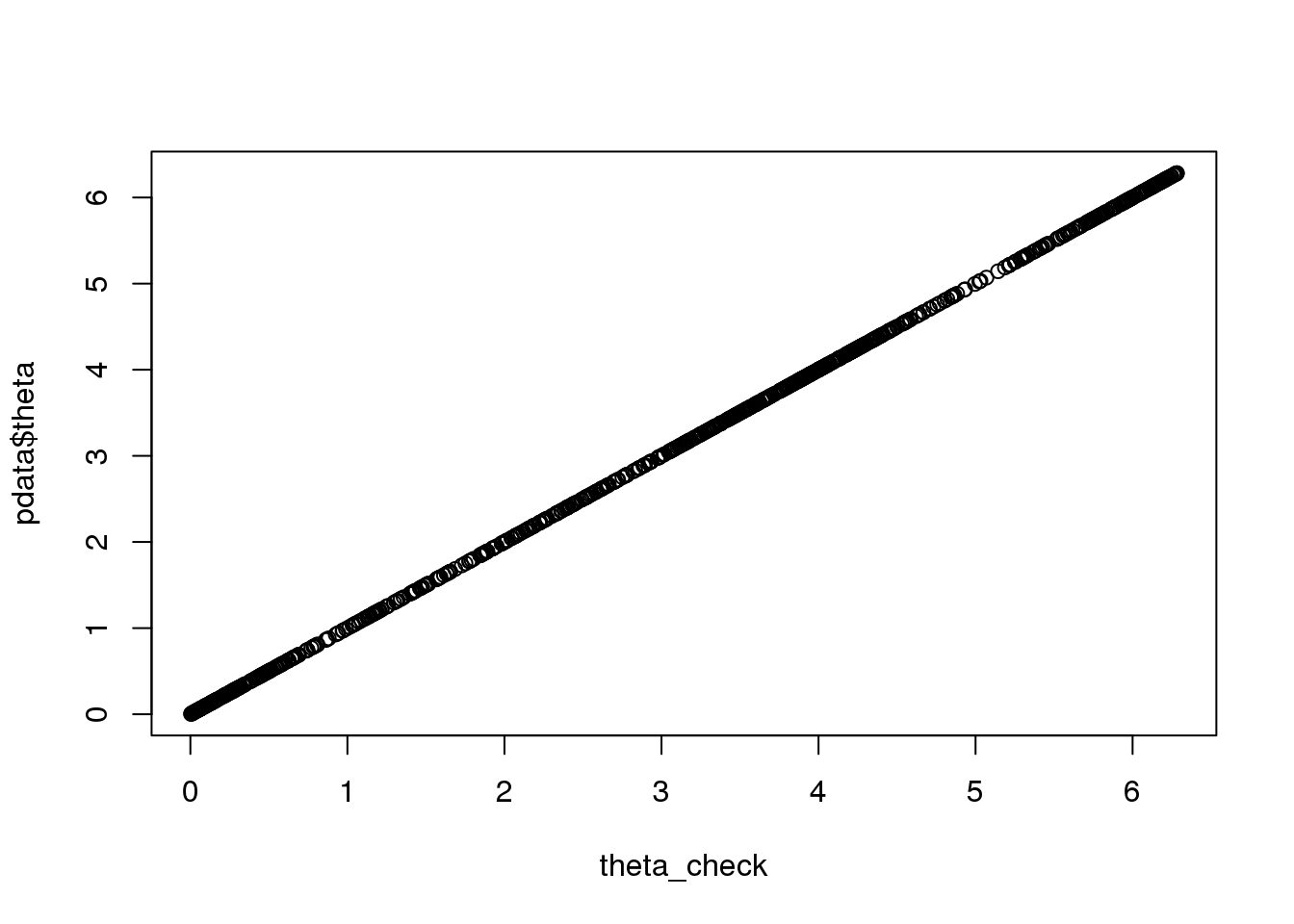
names(theta_check) <- rownames(pdata)
dist_to_origin <- sqrt(pca_df[,1]^2+pca_df[,2]^2)
which_out <- rownames(pdata)[which(scale(dist_to_origin) < -1)]
plot(pca_df[,1],
pca_df[,2],
col=c("gray50", "forestgreen")[(scale(dist_to_origin) < -1)+1], pch=16,
xlab="PC1",
ylab="PC2",
main="Distance-to-Origin < -1 SD")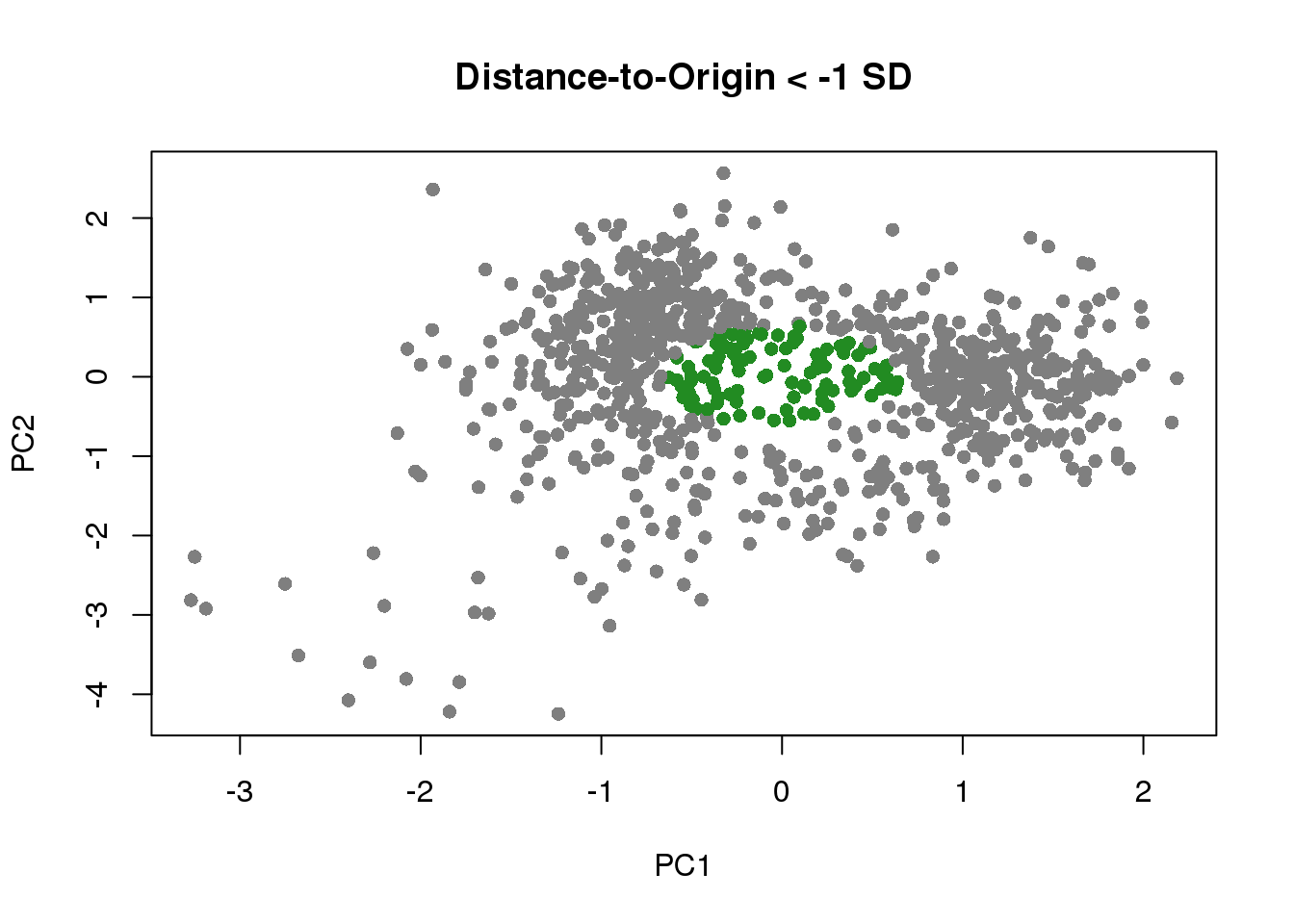
fitting top 10 genes without PC outliers
fits_sub_top10 <- vector("list", 5)
for (run in 1:5) {
print(run)
# fitting training data
Y_train <- expr.sig[,part_indices[[run]]$train]
theta_train <- theta_check[match(colnames(Y_train), rownames(pdata))]
names(theta_train) <- colnames(Y_train)
Y_train_sub <- Y_train[,which(!(colnames(Y_train) %in% which_out))]
theta_train_sub <- theta_train[which(!(names(theta_train) %in% which_out))]
fit.train <- cycle.npreg.insample(Y = Y_train_sub,
theta = theta_train_sub,
ncores=12,
polyorder=2,
method.trend="trendfilter")
# fitting test data
Y_test <- expr.sig[,part_indices[[run]]$test]
theta_test <- theta_check[match(colnames(Y_test), rownames(pdata))]
names(theta_test) <- colnames(Y_test)
Y_test_sub <- Y_test[,which(!(colnames(Y_test) %in% which_out))]
theta_test_sub <- theta_test[which(!(names(theta_test) %in% which_out))]
fit.test <- cycle.npreg.outsample(Y_test=Y_test_sub,
sigma_est=fit.train$sigma_est,
funs_est=fit.train$funs_est,
method.grid = "uniform",
method.trend="trendfilter",
ncores=12)
fits_sub_top10[[run]] <- list(fit.train=fit.train,
fit.test=fit.test,
theta_test=theta_test_sub)
}
for (i in 1:5) {
fits_sub_top10[[i]]$theta_est_shift <- rotation(fits_sub_top10[[i]]$theta_test, fits_sub_top10[[i]]$fit.test$cell_times_est)$y2shift
}
saveRDS(fits_sub_top10, file = "../output/method-train-labels.Rmd/fits_sub_top10.rds")
diff_time <- lapply(1:5, function(i) {
pmin(abs(fits_sub_top10[[i]]$theta_est_shift-fits_sub_top10[[i]]$theta_test),
abs(fits_sub_top10[[i]]$theta_est_shift-(2*pi-fits_sub_top10[[i]]$theta_test)))
})
pve <- lapply(1:length(fits_sub), function(i) {
dap <- pdata$dapi.median.log10sum[match(names(fits_sub_top10[[i]]$theta_test),rownames(pdata))]
get.pve(dap[order(fits_sub_top10[[i]]$theta_est_shift)])
})
save(diff_time, pve,
file="../output/method-train-labels.Rmd/modelresults_excludeoutlier_top10.rda")load(file="../output/method-train-labels.Rmd/modelresults_excludeoutlier_top10.rda")
mean(sapply(diff_time, mean)/2/pi)[1] 0.08253012mean(unlist(pve))[1] 0.2916538Cell time properties before/after removing PC outliers (intensity PVE)
rfp_theta <- with(pdata,
get.pve(rfp.median.log10sum.adjust[order(theta)]))
gfp_theta <- with(pdata,
get.pve(gfp.median.log10sum.adjust[order(theta)]))
dapi_theta <- with(pdata,
get.pve(dapi.median.log10sum.adjust[order(theta)]))
save(rfp_theta,
gfp_theta,
dapi_theta,
file = "../output/method-train-labels.Rmd/pve_include_pc_outlier.rda")
rfp_theta_sub <- with(pdata[which(!(rownames(pdata) %in% which_out)),],
get.pve(rfp.median.log10sum.adjust[order(theta)]))
gfp_theta_sub <- with(pdata[which(!(rownames(pdata) %in% which_out)),],
get.pve(gfp.median.log10sum.adjust[order(theta)]))
dapi_theta_sub <- with(pdata[which(!(rownames(pdata) %in% which_out)),],
get.pve(dapi.median.log10sum.adjust[order(theta)]))
save(rfp_theta_sub,
gfp_theta_sub,
dapi_theta_sub,
file = "../output/method-train-labels.Rmd/pve_no_pc_outlier.rda")load(file="../output/method-train-labels.Rmd/pve_no_pc_outlier.rda")
c(rfp_theta_sub,
gfp_theta_sub,
dapi_theta_sub) $pve
[1] 0.8920721
$pval
[1] 0
$pve
[1] 0.7175765
$pval
[1] 0
$pve
[1] 0.2673241
$pval
[1] 1.4491e-05load(file="../output/method-train-labels.Rmd/pve_include_pc_outlier.rda")
c(rfp_theta$pve,
gfp_theta$pve,
dapi_theta$pve) [1] 0.8694718 0.6988123 0.2274918Re-fitting after removing PC outliers from the same 5 folds, top 101 genes
expr_sub <- log2cpm.quant.nonvalid[rownames(log2cpm.quant.nonvalid) %in% rownames(sig.genes), ]
fits_sub_top101 <- vector("list", 5)
for (run in 1:5) {
print(run)
# fitting training data
Y_train <- expr_sub[,part_indices[[run]]$train]
theta_train <- theta_check[match(colnames(Y_train), rownames(pdata))]
names(theta_train) <- colnames(Y_train)
Y_train_sub <- Y_train[,which(!(colnames(Y_train) %in% which_out))]
theta_train_sub <- theta_train[which(!(names(theta_train) %in% which_out))]
fit.train <- cycle.npreg.insample(Y = Y_train_sub,
theta = theta_train_sub,
ncores=20,
method.trend="trendfilter")
# fitting test data
Y_test <- expr_sub[,part_indices[[run]]$test]
theta_test <- theta_check[match(colnames(Y_test), rownames(pdata))]
names(theta_test) <- colnames(Y_test)
Y_test_sub <- Y_test[,which(!(colnames(Y_test) %in% which_out))]
theta_test_sub <- theta_test[which(!(names(theta_test) %in% which_out))]
fit.test <- cycle.npreg.outsample(Y_test=Y_test_sub,
sigma_est=fit.train$sigma_est,
funs_est=fit.train$funs_est,
method.grid = "uniform",
method.trend="trendfilter",
polyorder=2,
ncores=20)
fits_sub_top101[[run]] <- list(fit.train=fit.train,
fit.test=fit.test,
theta_test=theta_test_sub)
}
for (i in 1:5) {
fits_sub_top101[[i]]$theta_est_shift <- rotation(fits_sub_top101[[i]]$theta_test, fits_sub_top101[[i]]$fit.test$cell_times_est)$y2shift
}
saveRDS(fits_sub_top101, file = "../output/method-train-labels.Rmd/fits_sub_top101.rds")
diff_time <- lapply(1:5, function(i) {
pmin(abs(fits_sub_top101[[i]]$theta_est_shift-fits_sub_top101[[i]]$theta_test),
abs(fits_sub_top101[[i]]$theta_est_shift-(2*pi-fits_sub_top101[[i]]$theta_test)))
})
pve <- lapply(1:length(fits_sub_top101), function(i) {
dap <- pdata$dapi.median.log10sum[match(names(fits_sub_top101[[i]]$theta_test),
rownames(pdata))]
get.pve(dap[order(fits_sub_top101[[i]]$theta_est_shift)])
})
save(diff_time, pve,
file="../output/method-train-labels.Rmd/modelresults_excludeoutlier_top101.rda")load(file="../output/method-train-labels.Rmd/modelresults_excludeoutlier_top101.rda")
mean(sapply(diff_time, mean)/2/pi)[1] 0.09344773mean(unlist(pve))[1] 0.2832532Fitting including PC outliers
expr_sub <- log2cpm.quant.nonvalid[rownames(log2cpm.quant.nonvalid) %in% rownames(sig.genes), ]
fits_top101 <- vector("list", 5)
for (run in 1:5) {
print(run)
# fitting training data
Y_train <- expr_sub[,part_indices[[run]]$train]
theta_train <- theta_check[match(colnames(Y_train), rownames(pdata))]
names(theta_train) <- colnames(Y_train)
# Y_train_sub <- Y_train[,which(!(colnames(Y_train) %in% which_out))]
# theta_train_sub <- theta_train[which(!(names(theta_train) %in% which_out))]
fit.train <- cycle.npreg.insample(Y = Y_train,
theta = theta_train,
ncores=12,
polyorder=2,
method.trend="trendfilter")
# fitting test data
Y_test <- expr_sub[,part_indices[[run]]$test]
theta_test <- theta_check[match(colnames(Y_test), rownames(pdata))]
names(theta_test) <- colnames(Y_test)
# Y_test_sub <- Y_test[,which(!(colnames(Y_test) %in% which_out))]
# theta_test_sub <- theta_test[which(!(names(theta_test) %in% which_out))]
fit.test <- cycle.npreg.outsample(Y_test=Y_test,
sigma_est=fit.train$sigma_est,
funs_est=fit.train$funs_est,
method.grid = "uniform",
method.trend="trendfilter",
ncores=12)
fits_top101[[run]] <- list(fit.train=fit.train,
fit.test=fit.test,
theta_test=theta_test)
}
for (i in 1:5) {
fits_top101[[i]]$theta_est_shift <- rotation(fits_top101[[i]]$theta_test, fits_top101[[i]]$fit.test$cell_times_est)$y2shift
}
saveRDS(fits_top101, file = "../output/method-train-labels.Rmd/fits_top101.rds")
#fits_top101 <- readRDS(file = "../output/method-train-labels.Rmd/fits_top101.rds")
diff_time <- lapply(1:5, function(i) {
pmin(abs(fits_top101[[i]]$theta_est_shift-fits_top101[[i]]$theta_test),
abs(fits_top101[[i]]$theta_est_shift-(2*pi-fits_top101[[i]]$theta_test)))
})
pve <- lapply(1:length(fits_top101), function(i) {
dap <- pdata$dapi.median.log10sum.adjust[match(names(fits_top101[[i]]$theta_test),rownames(pdata))]
get.pve(dap[order(fits_top101[[i]]$theta_est_shift)])
})
save(diff_time, pve,
file = "../output/method-train-labels.Rmd/modelresults_top101.rda")load(file = "../output/method-train-labels.Rmd/modelresults_top101.rda")
mean(sapply(diff_time, mean)/2/pi)[1] 0.08756486mean(sapply(pve, "[[", 1))[1] 0.1411442Session information
sessionInfo()R version 3.4.3 (2017-11-30)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] parallel stats graphics grDevices utils datasets methods
[8] base
other attached packages:
[1] circular_0.4-93 Biobase_2.38.0 BiocGenerics_0.24.0
loaded via a namespace (and not attached):
[1] Rcpp_0.12.17 mvtnorm_1.0-8 digest_0.6.15 rprojroot_1.3-2
[5] backports_1.1.2 git2r_0.21.0 magrittr_1.5 evaluate_0.10.1
[9] stringi_1.1.6 boot_1.3-20 rmarkdown_1.10 tools_3.4.3
[13] stringr_1.2.0 yaml_2.1.16 compiler_3.4.3 htmltools_0.3.6
[17] knitr_1.20 This R Markdown site was created with workflowr